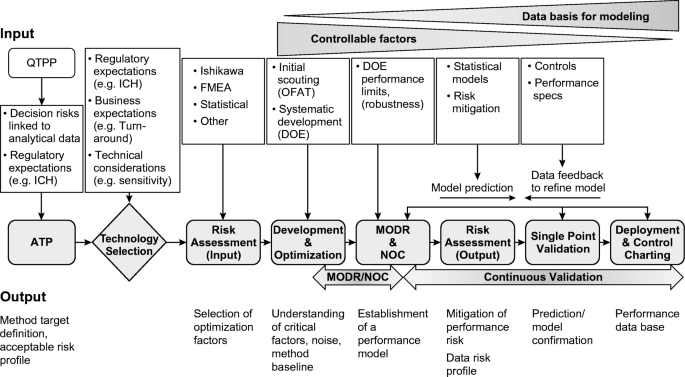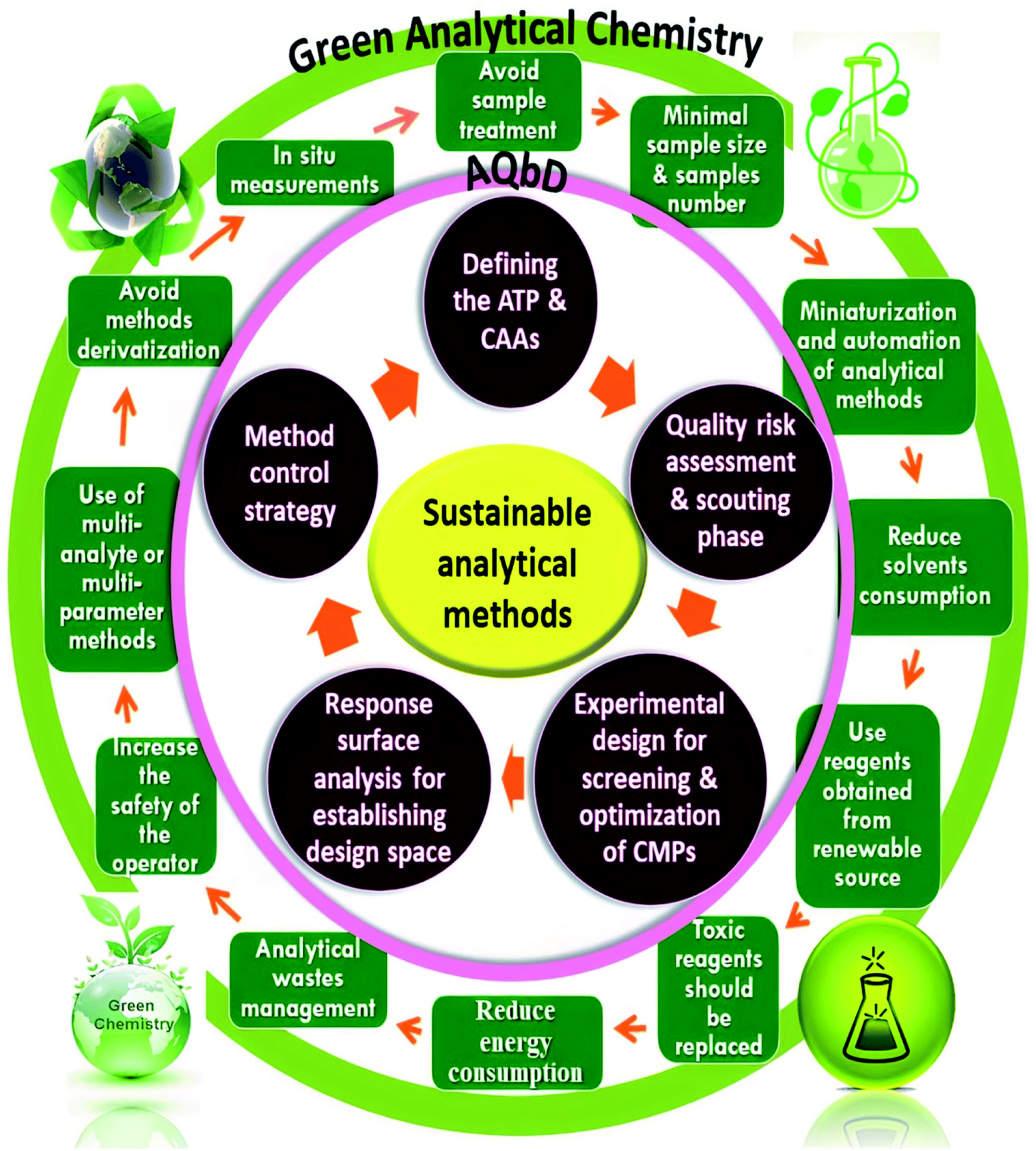
Quality by design approach for green HPLC method development for simultaneous analysis of two thalassemia drugs in biological fluid with pharmacokinet ... - RSC Advances (RSC Publishing) DOI:10.1039/D2RA00966H

An analytical quality by design (aQbD) approach for a l-asparaginase activity method - ScienceDirect

Analytical Quality by Design Based Method Development for the Analysis of Valsartan and Nitrosamines Impurities Using UPLC-MS | Waters

Development and Optimization of Liquid Chromatography Analytical Methods by Using AQbD Principles: Overview and Recent Advances | Organic Process Research & Development

Figure 1 from Analytical Quality by Design Approach to Test Method Development and Validation in Drug Substance Manufacturing | Semantic Scholar
![PDF] Analytical Quality by Design Approach to Test Method Development and Validation in Drug Substance Manufacturing | Semantic Scholar PDF] Analytical Quality by Design Approach to Test Method Development and Validation in Drug Substance Manufacturing | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/feb17b38a6e43d7096393c341016bfb3543f64cf/2-Table1-1.png)
PDF] Analytical Quality by Design Approach to Test Method Development and Validation in Drug Substance Manufacturing | Semantic Scholar

Maximize lab productivity and quality by embracing expected ICH Q14 Enhanced Method Development Guidelines and AQbD approaches Registration

Figure 2 from Quality Improvement with Scientific Approaches (QbD, AQbD and PAT) in Generic Drug Substance Development: Review | Semantic Scholar
Examining the basic principles of quality by design (QbD) approach in analytical studies. - Document - Gale Academic OneFile
Analytical method development by using QbD - An emerging approach for robust analytical method development
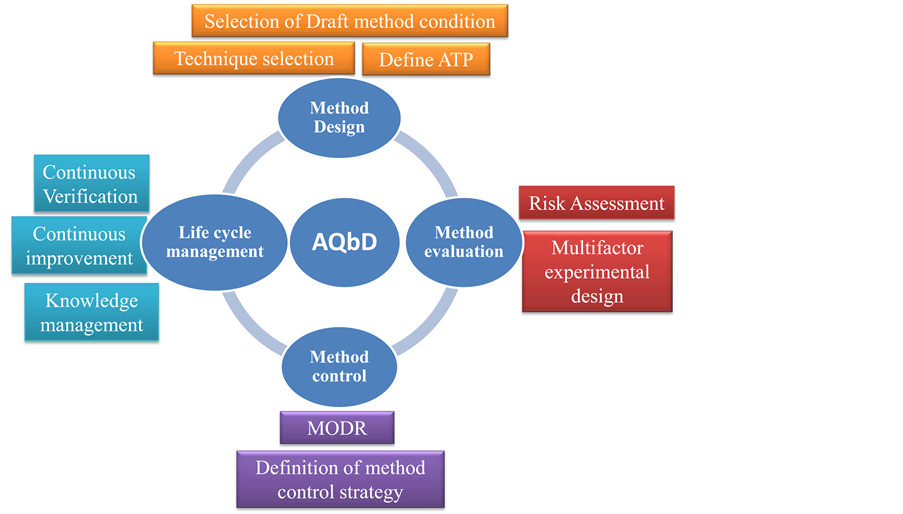
QbD Approach Method Development for Estimation of Dabigatran Etexilate along with Its Impurities and Identification of Degradants in Capsule Dosage Form